How chemotactic cells extend response range
Part of mechanism behind how organisms adapt to environment elucidated
Professor Ueda’s research group at Osaka University and Riken QBiC discovered a new protein factor that regulates the response range of eukaryotic chemotaxis and named the factor Gip1 (trimeric G protein interacting protein 1). Gip1 extends chemotactic range by regulating the intracellular distribution of G proteins that is a new regulatory mechanism for G proteins signaling.
Chemotaxis is directed cell migration and provides a unique spatio-temporally regulated signal transduction system to control early embryogenesis, neuronal wiring, wound healing, immune responses, and so on. Chemotactic cells share basic properties including high sensitivity to shallow gradients and responsiveness to a wide dynamic range of chemoattractants. The group identified a novel regulator of trimeric G protein as Gip1, which extends response range in chemotaxis. Their findings show three steps of Gip1 mediated response range extension. First, Gip1 sequesters the cytosolic pools of G proteins through binding, where cytosolic G proteins are not available for chemotactic signaling on the membrane. Second, upon stimulation with chemoattractant, G proteins dissociate from Gip1 and move to the cell membrane. Third, Gip1-dependent translocation supplies a signal transducer for chemoattractant receptors at higher ranges and causes a biased redistribution of G proteins on the membrane along chemical gradients. This biased redistribution contributes to proper gradient sensing at higher concentration ranges. These findings advance our knowledge of the molecular basis for wide-range sensing and is distinct from the chemical modification-based mechanisms of sensory receptors. This mechanism could be harnessed for broad dynamic range regulation that is a frequently observed feature of sensory cells.
Abstract Chemotactic eukaryote cells can sense chemical gradients over a wide range of concentrations via heterotrimeric G-protein signaling; however, the underlying wide-range sensing mechanisms are only partially understood. Here we report that a novel regulator of G proteins, G protein-interacting protein 1 (Gip1), is essential for extending the chemotactic range of Dictyostelium cells. Genetic disruption of Gip1 caused severe defects in gradient sensing and directed cell migration at high but not low concentrations of chemoattractant. Also, Gip1 was found to bind and sequester G proteins in cytosolic pools. Receptor activation induced G-protein translocation to the plasma membrane from the cytosol in a Gip1-dependent manner, causing a biased redistribution of G protein on the membrane along a chemoattractant gradient. These findings suggest that Gip1 regulates G-protein shuttling between the cytosol and the membrane to ensure the availability and biased redistribution of G protein on the membrane for receptor-mediated chemotactic signaling. This mechanism offers an explanation for the wide-range sensing seen in eukaryotic chemotaxis.
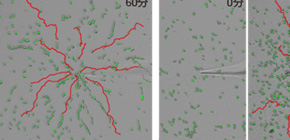
Dictyostelium cells normally migrate (right) toward a high concentration of cAMP signal (red dot), but Dictyostelium cells lacking the Gip1 protein (left)
lose their capacity to respond to such cues.
To learn more about this research, please view the full research report entitled " Heterotrimeric G protein shuttling via Gip1 extends the dynamic range of eukaryotic chemotaxis " at this page of the Proceedings of National Academy of Science of the United States of America website.
Related link
Stochastic Biocomputing Group, Graduate School of Frontier Biosciences, Osaka University (link in Japanese)
http://www.bio.sci.osaka-u.ac.jp/bio_web/lab_page/ueda/
