Elucidation of how abnormalities in intracellular protein trafficking interfere with higher brain functions
Will lead to the development of new therapeutic medicines for neuropsychiatric disorders
A group of researchers led by NAKAZAWA Takanobu (Specially Appointed Associate Professor, Graduate School of Pharmaceutical Sciences, Osaka University), KANO Masanobu (Professor, Graduate School of Medicine, The University of Tokyo), and HASHIMOTO Ryota (Associate Professor, United Graduate School of Child Development, Osaka University, Kanazawa University, Hamamatsu University School of Medicine, Chiba University and University of Fukui) found the mechanism for regulating synaptic functions via intracellular protein trafficking and revealed that abnormalities in intracellular protein trafficking interfere with higher brain functions such as memory and learning.
This joint research group elucidated that ARHGAP33 regulates synaptic functions via intracellular protein trafficking and that the lack of ARHGAP33 causes abnormal higher brain functions. This group also found that abnormalities in intracellular protein trafficking are is blamed in part on the onset of neuropsychiatric disorders such as schizophrenia.
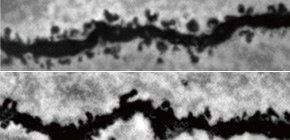
This group created ARHGAP33 knock-out (KO) mice in order to examine the function of ARHGAP33, which is common in the brain, and abnormalities were found in these ARHGAP33 KO mice. The small size and low frequency of miniature excitatory postsynaptic currents showed an abnormality in synaptic function as well.
This group also examined higher brain functions in ARHGAP33 KO mice and found a working memory disorder and abnormalities in prepulse inhibition, the latter being is related to information processing in the brain.
The possibility that ARHGAP33 KO mice cannot remember the path they had just taken was found. They also showed little startle response to sound, which suggested impaired information processing in the brain.
This group also found the molecular mechanism explaining that ARHGAP33 regulates synapse formation and memory; that is, ARHGAP33 at the Golgi apparatus of neurons are involved in the trafficking of neurotrophin receptors, or Tropomyosin receptor kinase B (TrkB), to synaptic sites.
Neurotrophin is a factor necessary for formation and function of synapses. It is thought that TrkB is not transported to synaptic sites due to the lack of ARHGAP33, which decreases synapse function, resulting in abnormalities in higher brain functions.
Most causes of the onset of neuropsychiatric disorders are still not well understood, and the development of new therapeutic medicines is an urgent issue. In such a setting, this group’s achievement of elucidating the molecular mechanism of abnormalities in higher brain functions, which are related to neuropsychiatric disorders, is highly noteworthy in the fields of psychiatric medicine, basic medicine, and pharmacy. This group’s achievement could lead to the development of new therapeutic medicines for neuropsychiatric disorders, such as schizophrenia.
Abstract
Intracellular trafficking of receptor proteins is essential for neurons to detect various extracellular factors during the formation and refinement of neural circuits. However, the precise mechanisms underlying the trafficking of neurotrophin receptors to synapses remain elusive. Here, we demonstrate that a brain-enriched sorting nexin, ARHGAP33, is a new type of regulator for the intracellular trafficking of TrkB, a high-affinity receptor for brain-derived neurotrophic factor. ARHGAP33 knockout (KO) mice exhibit reduced expression of synaptic TrkB, impaired spine development and neuropsychiatric disorder-related behavioural abnormalities. These deficits are rescued by specific pharmacological enhancement of TrkB signalling in ARHGAP33 KO mice. Mechanistically, ARHGAP33 interacts with SORT1 to cooperatively regulate TrkB trafficking. Human ARHGAP33 is associated with brain phenotypes and reduced SORT1 expression is found in patients with schizophrenia. We propose that ARHGAP33/SORT1-mediated TrkB trafficking is essential for synapse development and that the dysfunction of this mechanism may be a new molecular pathology of neuropsychiatric disorders.
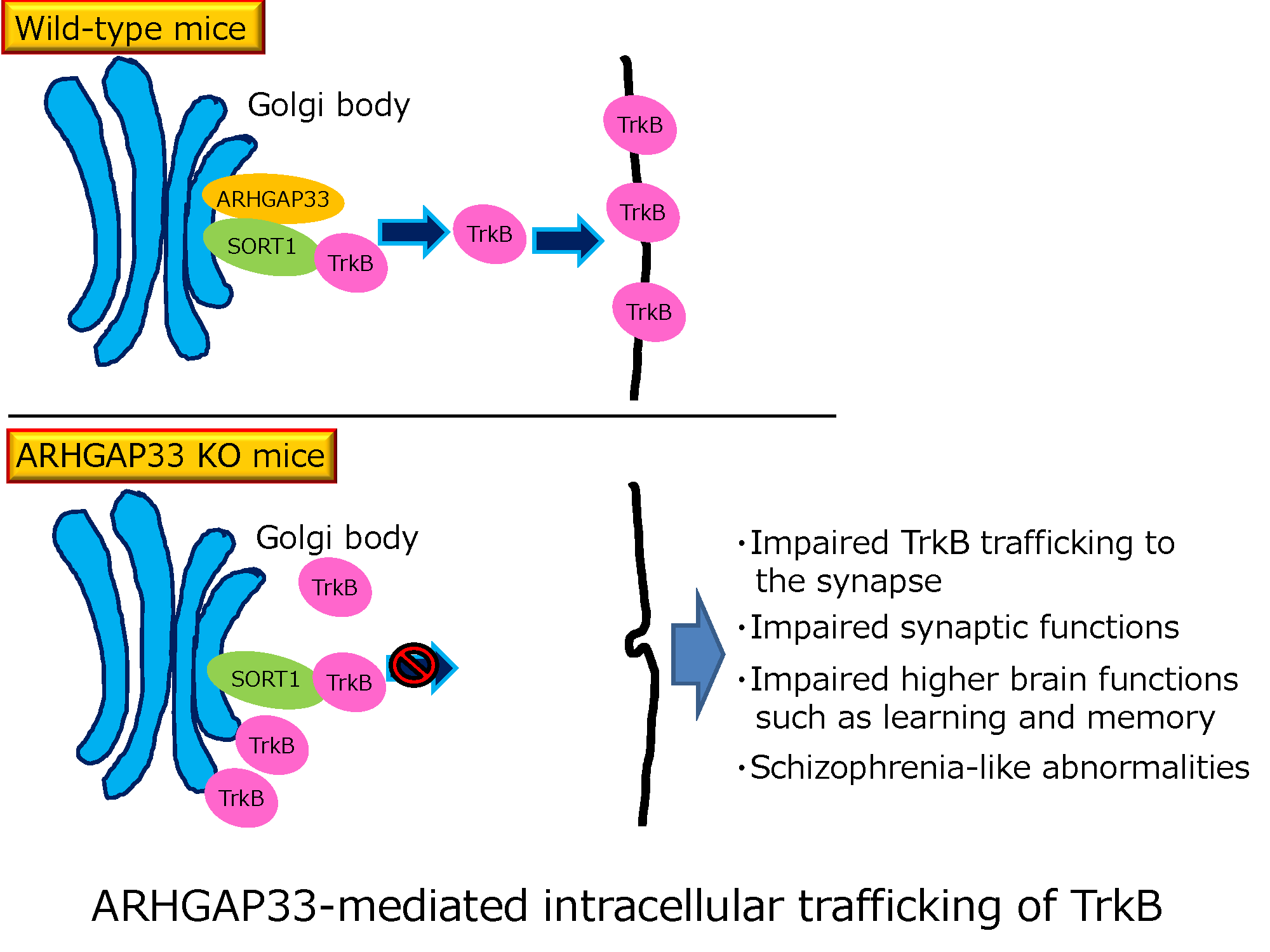
Figure 1

Figure 2
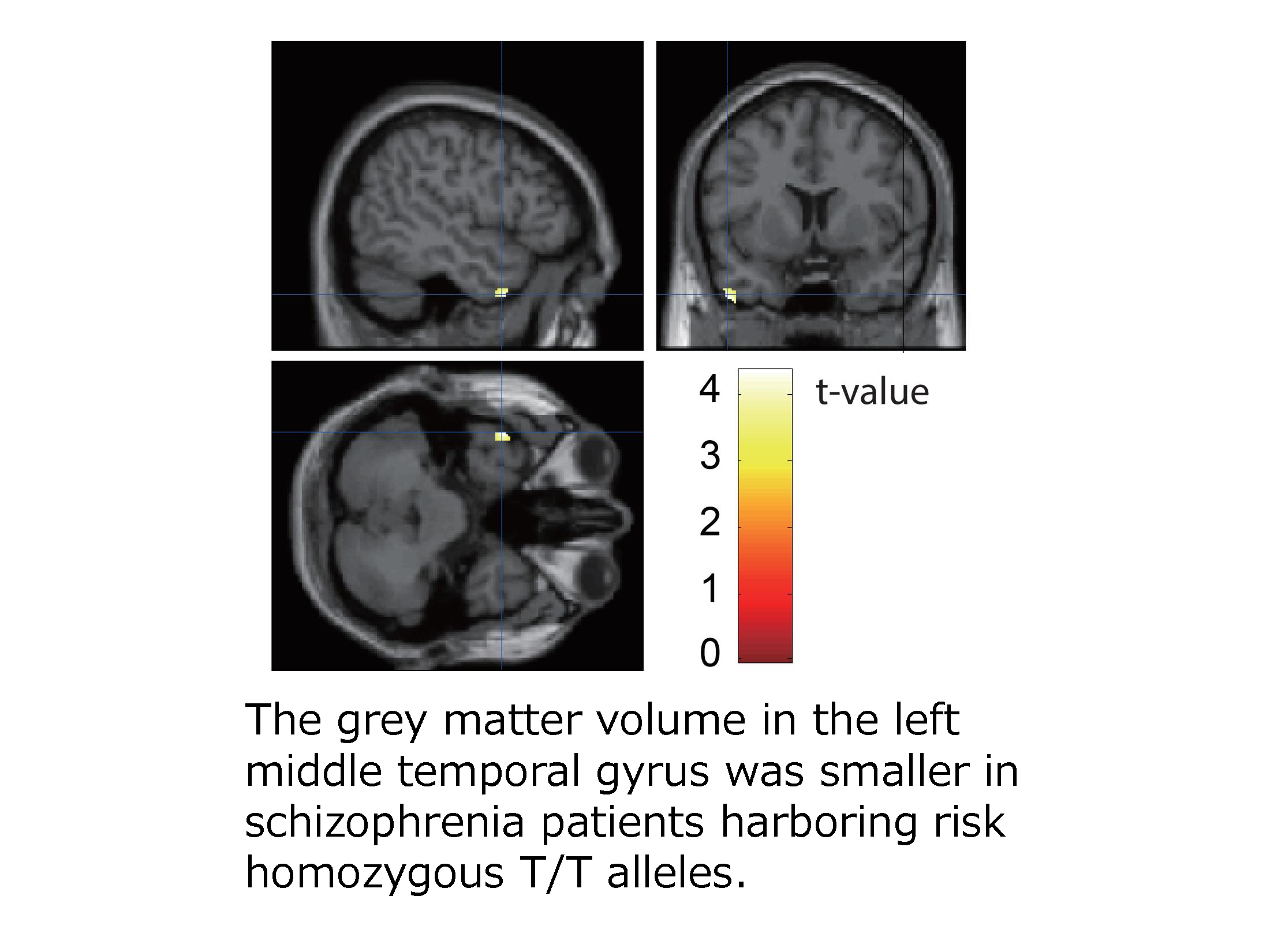
Figure 3
To learn more about this research, please view the full research report entitled " Emerging roles of ARHGAP33 in intracellular trafficking of TrkB and pathophysiology of neuropsychiatric disorders " at this page of the Nature Communications website.
Related links
