Mystery of distorted-chair structure in photosynthesis clarified
Major step in realization of artificial photosynthesis
In photosynthesis in algae and higher plants, water is split into oxygen gas and hydrogen ions.
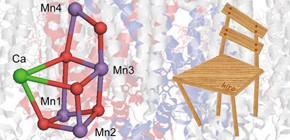
In Mn4CaO5, a complex, natural catalyst in photosynthetic protein Photosystem II (PSII), the bonds between manganese (Mn) and oxygen (O) atoms are elongated causing it to have a structure resembling that of a distorted chair. It is known that the distortion plays an important role in catalytic activity in water splitting; however, the snail-paced clarification of what role the distortion plays has hindered the development of efficient artificial photosynthesis.
A group of researchers led by Professor Hiroshi ISHIKITA and Assistant professor Keisuke SAITO (Graduate School of Science, Osaka University), by employing combined quantum mechanics/molecular mechanics (QM/MM) calculation methods, succeeded in revising the accepted idea that the presence of Ca distorted the structure. (This year's Nobel Prize in Chemistry was awarded for QM/MM calculation.)
This group has clarified that the direct cause of the distortion was not Ca located in the seat of the chair-like structure, but Mn located in the back of the chair-like structure.
This achievement is expected to accelerate the development of artificial photosynthesis.
Professor Hiroshi ISHIKITA, conducted research at the University of Southern California through 2008 as a post-graduate researcher for this year's Nobel Prize in Chemistry awardee Professor Arieh Warshel.
Abstract
In the crystal structure of Photosystem II (PSII) analyzed at a resolution of 1.9 Å, most of the bond lengths between Mn and O atoms in the oxygen-evolving Mn4Ca cluster are 1.8–2.1 Å. On the other hand, the Mn1single bondO5 bond in the Mn3CaO4 cubane region of the Mn4Ca cluster is significantly elongated to 2.6 Å. Using a quantum mechanical/molecular mechanical approach, we investigated factors that are responsible for distortion of the Mn3CaO4 cubane. Removal of Ca led to shortening the Mn1single bondO5 bond by 0.2 Å; however, Mn1single bondO5 remained significantly elongated, at > 2.5 Å. Conversely, removal of Mn4 significantly shortens the Mn1single bondO5 distance by 0.5 Å to 2.2 Å, resulting in a more symmetric cubane shape. These results suggest that Mn4, not Ca, is predominantly responsible for distortion of the Mn3CaO4 cubane. It was not the Ca component that was responsible for the existence of the two S2 conformers but two different Mn oxidation states (Mn1, Mn2, Mn3, M4) = (III, IV, IV, IV) and (IV, IV, IV, III); they were interconvertible by translocation of the O5 atom along the Mn1–O5–Mn4 axis. Depletion of Ca resulted in rearrangement of the H-bond network near TyrZ, which proceeds via a chloride ion (Cl-1 pathway). This may explain why Ca depletion inhibits the S2 to S3 transition, the same process that can also be inhibited by Cl− depletion.

Figure 1

Figure 2

Figure 3
To learn more about this research, please read the full research report entitled " Influence of the Ca 2+ ion on the Mn 4 Ca conformation and the H-bond network arrangement in Photosystem II " at this page of the Science Direct website.
Related link :
