Modified enzymes for bio-hydrogen production
Genji KURISU, Professor, Institute for Protein Research
Changing the cofactors that help proteins work in a search for hybrid enzymes that could fuel the hydrogen economy
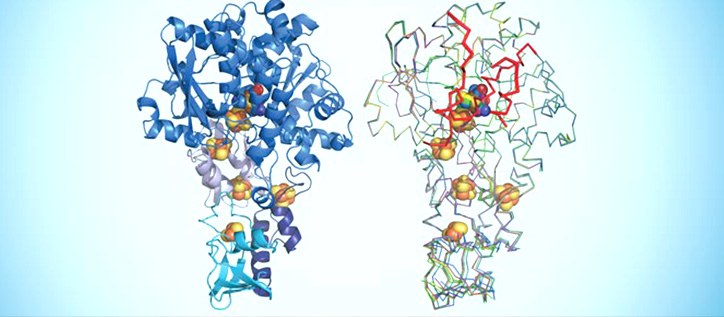
Credit: Reproduced from Ref. 1 and licensed under CC BY 3.0 © 2015 J Esselborn et al.[ http://creativecommons.org/licenses/by/3.0/ ]
Caption: Schematic representations of semisynthetic proteins that can catalyze the production of hydrogen with the help of metallic cofactor atoms (colored spheres).
Modified enzymes for bio-hydrogen production
Changing the cofactors that help proteins work in a search for hybrid enzymes that could fuel the hydrogen economy
A collaboration between researchers at Osaka University in Japan and Ruhr University Bochum in Germany is investigating enzymes that can catalyze fast and efficient production of hydrogen. The researchers are exploring how these enzymes might be modified and adapted to produce hydrogen on an industrial scale.
“The enzymes we are studying might be used to produce bio-hydrogen,” explains Genji Kurisu of Osaka University’s Institute for Protein Research. This would mean tweaking photosynthesis in algae to optimize the process of using sunlight to split water into oxygen and hydrogen. However, Kurisu cautions that years of basic research will probably be necessary before their work could move to an industrial scale.
The enzymes, known as [FeFe]-hydrogenases, are found in simple organisms such as bacteria and algae and are nature’s fastest catalysts for producing hydrogen. Kurisu is collaborating with the research group in Germany, led by Thomas Happe, to learn more about the chemistry of these natural enzymes, and also how semi-synthetic modified forms could be made more stable and effective.
[FeFe]-hydrogenases are ‘metalloproteins’ that must have a small chemical cofactor containing iron bound to the protein molecules to allow them to perform their impressive feats of catalysis. Kurisu has been working with metalloproteins since his doctoral research project in the 1990s. With colleagues at Osaka, he has become skilled in determining their precise three-dimensional structures.
Kurisu’s interests in the fundamental structure and basic science of the enzyme activity perfectly complements Happe’s particular interest in the effects of replacing natural cofactors with synthetic ones toward applications for modified enzymes. “This is a really good collaboration for us,” says Kurisu.
In their most recent investigation, the collaborators examined the precise structural changes that occur when synthetic cofactors are incorporated into the enzymes (see image)[1]. They achieve this by making crystals of the semisynthetic enzymes and using a beam of X-rays to reveal their structure — a technique called X-ray crystallography. “Osaka has opened protein crystallography as an important technique for me, which will influence the topics I can work on,” comments Happe.
Through their investigation the team identified the essential parts of the cofactors needed for enzyme activity. This is difficult work, because the enzymes come from organisms that function under anaerobic conditions. “Purifying and crystallizing the enzymes using an oxygen-free glove box can be very stressful,” says Kurisu.
With the ever-increasing interest in hydrogen as a renewable fuel, clearing all these hurdles may eventually help to build a clean and sustainable hydrogen-based economy.
Related link
Laboratory of Protein Crystallography, Osaka University
http://www.protein.osaka-u.ac.jp/crystallography/EngHP/Home.html

Professor Genji Kurisu
Laboratory of Protein Crystallography
Institute for Protein Research
Osaka University
gkurisu@protein.osaka-u.ac.jp
Reference:
1. Esselborn, J., Muraki, N., Klein, K., Engelbrecht, V. Metzler-Holte, N. et al. A structural view of synthetic cofactor integration into [FeFe]-hydrogenases. Chemical Science 7, 959–968 (2015).
DOI: http://doi.org/10.1039/C5SC03397G
This research project was supported by the Osaka University International Joint Research Promotion Program, which aims to further enhance research quality and promote globalization at Osaka University through advanced research with overseas collaborators. Professor Kurisu jointly conducted this research with the following researchers: Professor Thomas Happe, Ruhr University Bochum, Germany
Interview held in March 2016
