
Electron Accelerators Reveal the Radical Secrets of Antioxidants
Osaka University researcher proves the value of electron accelerators for creating free radicals and understanding their damaging effects on biological molecules
In a groundbreaking series of experiments, an Osaka University researcher has demonstrated an exciting new method for understanding the power of antioxidants to protect us from harmful free radicals. Professor Kazuo Kobayashi has used linear electron accelerators, sometimes called “linacs,” to fling electrons at speeds not previously seen in biological research. When the electrons slammed into water molecules in the samples, highly reactive free radicals were produced. This work will be extremely valuable for understanding the body’s naturally occurring antioxidant molecules and proteins, such as ascorbic acid, also called vitamin C.
A free radical is a molecule with an unpaired electron, which makes it very eager to react. Some biological processes, including photosynthesis, harness energic free radicals to power vital chemical reactions. However, when a free radical gets loose, it can be extremely damaging to DNA and other important biomolecules. Rogue radicals can also be created by radiation, including from the sun’s UV light. To avert damage from free radicals, a circulating antioxidant molecule or protein in the body can absorb the extra electron. For many years, scientists could only guess at the exact pathway of this process, since the transfer of the electron from the free radical to the antioxidant occurs extremely fast, in times measured in trillionths of a second.
In the current research, to watch the charge transfer in action, electrons were accelerated by a linac in a process called pulse radiolysis. Since biological samples almost always contain water, the electrons could be counted on to slam into H2O molecules, leading to the rapid and reliable generation of free radicals inside the sample. Although the merits of this innovation are widely applicable, it took many years to gain acceptance in biological fields.
“Linacs are well-known in the field chemistry and physics,” Professor Kobayashi explains, “but less familiar to researchers from other fields. Some skeptics thought they are too complex and damaging to biomolecules to be useful. However, this research demonstrates how valuable linacs can be for understanding a wide range of biological processes.”
This method can not only elucidate many uncertain biological reaction mechanisms that include electron transfers, but also help develop new medications for preventing cell damage.
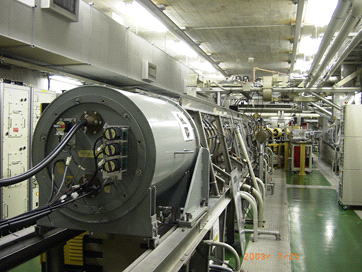
Fig.1. Linear electron accelerator, linac at The Institute of Scientific and Industrial Research, Osaka University
(credit: Osaka University)
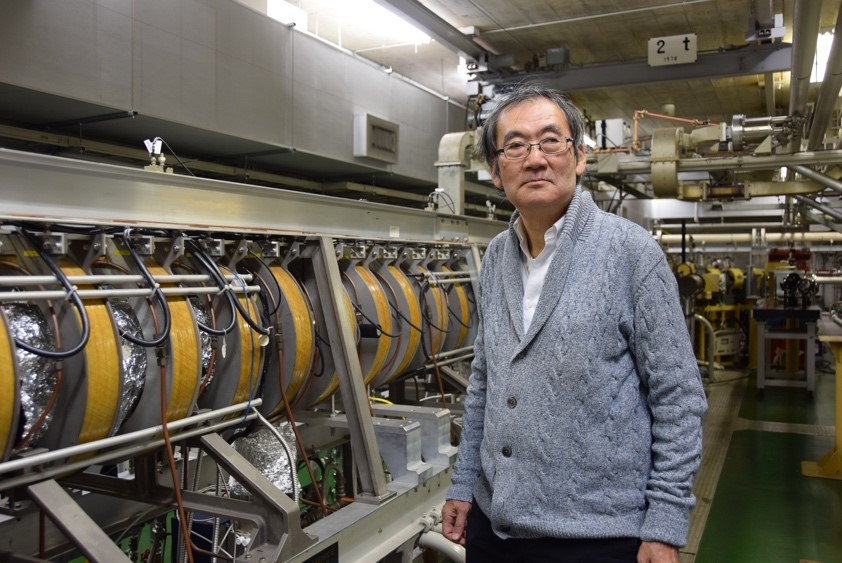
Fig.2. Professor Kazuo Kobayashi observes biochemical redox reactions on time scales ranging from picoseconds to seconds with a linac, a world first.
(credit: Osaka University)
The work is published in Chemical Reviews as “Pulse Radiolysis Studies for Mechanism in Biochemical Redox Reactions” at DOI: https://doi.org/10.1021/acs.chemrev.8b00405 .
Related links
