Real-time observation of DNA strand breaks in radioactive cancer therapy
“Hot” excited electrons play a role in bond breakage
A research group of researchers from Kyoto University, Oakland University (USA), Osaka University, The University of Tokyo, and Centre National de la Recherche Scientifique (CNRS, France) directly observed radiation-induced DNA damage, which occurs within 1 nanosecond (ns), a world first.
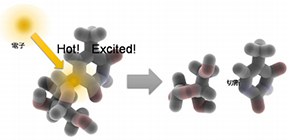
They demonstrated that electrons kicked out of water by radiation attached to ribothymidine (rT), a constituent of DNA and RNA, during their “hot” ground state (before getting into their “fully cooled” ground state), producing excited molecules, or free radicals, then causing bond breaking.
Cancer treatment using radiation is widely used as a minimally invasive therapy. When a human body is exposed to radiation, ionization, a process by which ions are formed by loss of an electron from a water molecule, is caused. To kill cancer cells, it is necessary to efficiently destroy the DNA of cancer cells by using ionization caused by radiation in the body.
When water is irradiated, ionization occurs. Negatively charged electrons slow down due to their interaction with water molecules, which orient themselves around electrons produced by ionization. The electrons produced by ionization of water become hydrated electrons within one picosecond. So, what chemical reactions were caused by electrons and how they broke DNA strains were not directly observed.
Using a picosecond pulse of a high energy electron beam, the researchers ionized diethylene glycol (DEG). Electron solvation events in DEG are slower than in water, occurring on the order of tens of picoseconds. The researchers ionized DEG using a picosecond pulse of a high energy electron beam to examine reactions triggered by electrons, finding a new reaction between DNA and electrons.
When irradiating 5-methyluridine, also called ribothymidine (rT), in liquid DEG, electrons kicked out of water were directly captured by rT much faster than before getting into a ground state, producing anion radicals in an excited state that had higher energy than the ground state. Because an excited rT anion radical exhibits a nearly concentration-independent decay with a time constant of about 350 ps, it was confirmed that the glycosidic bond cleavage in rT occurred via excited anion radical.
The results of this research will be helpful in developing chemical reaction control methods for preventing and repairing radiation–induced DNA damage in normal cells.
Abstract
Damage to DNA via dissociative electron attachment has been well-studied in both the gas and condensed phases; however, understanding this process in bulk solution at a fundamental level is still a challenge. Here, we use a picosecond pulse of a high energy electron beam to generate electrons in liquid diethylene glycol and observe the electron attachment dynamics to ribothymidine at different stages of electron relaxation. Our transient spectroscopic results reveal that the quasi-free electron with energy near the conduction band effectively attaches to ribothymidine leading to a new absorbing species that is characterized in the UV-visible region. This species exhibits a nearly concentration-independent decay with a time constant of ~350 ps. From time-resolved studies under different conditions, combined with data analysis and theoretical calculations, we assign this intermediate to an excited anion radical that undergoes N1-C1′ glycosidic bond dissociation rather than relaxation to its ground state.
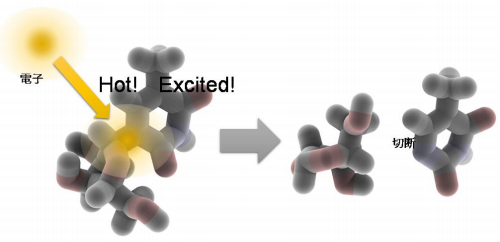
Figure 1

Figure 2
The article, “Observation of dissociative quasi-free electron attachment to nucleoside via excited anion radicalin solution: excited anion radical formation and its dissociation” was published in the Nature Communications at DOI: https://doi.org/10.1038/s41467-018-08005-z .
Related links
