Phase III clinical trials for stem cell-based cartilage regeneration therapy have started
A group of researchers at Osaka University developed a synthetic tissue using synovium-derived mesenchymal stem cells (MSCs) for treating damaged cartilage, which had previously been incurable and had no effective therapies.
Following the first in-human clinical trial at Osaka University Hospital, the surgery of the first patient was performed in the Phase III clinical study to confirm efficacy and safety of this therapy. This is the first clinical trial of regenerative therapy in Japan in terms of using allogeneic stem cells and the commercial use of stem cell bank at the Medical Center for Translational Research (MTR) *1 of Osaka University.
It is accepted that articular cartilage does not have blood supply and thus has very limited ability to heal itself, and there have been no effective treatment methods. Therapies using stem cells and tissue engineered techniques are being developed throughout the world; however, it was difficult to attain good regenerative repair quality and good tissue integrationtolesion base.
By the combination of monolayer culture and suspension culture, Norimasa NAKAMURA, Hideki YOSHIKAWA, and Yoshiki SAWA at Osaka University developed three-dimensional (3D) synthetic tissue with excellent differentiation ability and tissue adhesive properties by using only mesenchymal stem cells (MSCs) as the starting material. These unique properties have enabled transplantation by minimally invasive approaches such as arthroscopy ( Figure 1 ).
This tissue engineering technique is internationally unique to induce regenerative cartilage repair without using animal-derived materials and chemical compositions, which was patented in Japan and overseas.
This is the first clinical trial for regenerative tissue repair in which mega pharmaceutical companies have participated: Twocells Company, Ltd. *2 is involved in the clinical trial with the support of Chugai Pharmaceutical Co., Ltd. *3 .
In this clinical trial, allogeneic culture is performed in a serum-free culture medium (artificial medium). Therefore, only one operation is required, in contrast with the case of conventional autologous implantation that requires two operations to accomplish the treatment. This could be advantageous in reducing burden on patients and in cost effectiveness. The social reimbursement of this method will prove a boon to many patients with not only athletic injuries but also early phase of degenerative joint diseases. The number of patients with potential osteoarthritis (OA) is estimated to be about 30 million. It is hoped that this therapy could prevent the onset of OA patients in middle-aged generations.
Figure 1: Scaffold-free tissue engineered construct derived from synovial mesenchymal stem cells (credit: Osaka University)
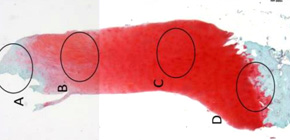
Figure 2: Cartilage repair by the TEC (Phase I/II trial). Top left: before implantation; Bottom left: 1yr post surgery; Right: Histology (credit: Osaka University)
The Medical Center for Translational and Clinical Research is Japan’s first translational research institute established in a university hospital in 2002. Since its establishment, this center has consistently supported research and development toward the commercialization of new innovative medical technologies by developing intellectual property strategies and supporting clinical development including drug production, non-clinical trials, pharmaceutical affairs, and investigator-initiated clinical trials. In particular, the cell culture preparation facilities, which are essential for the commercialization of regenerative medicine, are top-class in their scale, quality, number of projects, and operation rate, accepting the use of facilities for joint research with companies and clinical trials. In 2015, the Medical Center for Translational and Clinical Research established a cell bank, providing high-quality homologous cells which can be used for a consistent process from basic research to clinical application and commercialization.
Related links
