Successful synthesis of a new insulin analogue Selenoinsulin
Will lead to the development of long-lasting insulin preparations
A group of researchers led by Lecturer ARAI Kenta and Professor IWAOKA Michio (both from the Department of Chemistry, School of Science, Tokai University), Assistant Professor OKUMURA Masaki at the Frontier Research Institute for Interdisciplinary Sciences, Tohoku University, Assistant Professor WATANABE Satoshi and Professor INABA Kenji (both from the Institute of Multidisciplinary Research for Advanced Materials, Tohoku University), and HOJO Hironobu at the Institute for Protein Research, Osaka University, succeeded in developing synthetic insulin analogues selenoinsulin (Se-Ins) through the replacement of the interchain disulfide in bovine pancreatic insulin (BPIns) with a diselenide bridge.
This group demonstrated that Se-Ins had a nearly identical structure and bioactivity comparable to those of BPins, and that it had enhanced resistance to IDE degradation. This holds promise for application of Se-Ins to a long-lasting insulin preparation in diabetes therapy.
After circulating in the blood stream, insulin is degraded by an Insulin Degrading Enzyme (IDE) in the kidney and excreted in the urine. This group thought that the production of insulin with high resistance to degradation by IDE would lead to the development of a new type of long-lasting insulin preparations that could circulate in the body for a long time.
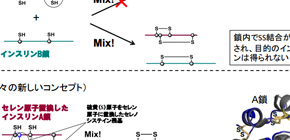
Insulin consists of two polypeptide chains: A chain and B chain, connected by a disulfide bond (Se−Se bond), so it’s difficult to take insulin only from these chains because of Se−Se bonds. Thus, this group thought that if insulin A-chains and B-chains containing selenium (Se) instead of sulfur (S), were used, the diselenide bond (Se−Se bond) would be formed quickly and then the chain-assembly reaction could be carried out efficiently, as Se is more reactive than S.
In addition, Se-Se bonds are more stable than S-S bonds, so this group anticipated that Se-Se bonds would give extra structural robustness on the insulin fold, thus resulting in the enhanced resistance to IDE degradation due to its intrinsic stability. Based on this concept, this group succeeded in the synthesis of Se-containing insulin A and B chains, as well as obtaining Se-Ins at the isolation yield of up to 27% by reacting peptide chains under optimal conditions.
This group demonstrated that Se-Ins had a nearly identical structure to that of BPIns, suggesting that Se-Ins has a bioactivity comparable to that of BPIns. Experiments of degradation of BPIns and Se-Ins by using IDE showed that the degradation rate of Se-Ins was much slower than that of BPIns. From this, it is thought that Se-Ins has a long-lasting nature and could be a new class of long-acting insulin analogues for diabetes therapy.
Abstract
Synthetic insulin analogues with a long lifetime are current drug targets for the therapy of diabetic patients. The replacement of the interchain disulfide with a diselenide bridge, which is more resistant to reduction and internal bond rotation, can enhance the lifetime of insulin in the presence of the insulin-degrading enzyme (IDE) without impairing the hormonal function. The [C7U A ,C7U B ] variant of bovine pancreatic insulin (BPIns) was successfully prepared by using two selenocysteine peptides (i.e., the C7U analogues of A- and B-chains, respectively). In a buffer solution at pH 10 they spontaneously assembled under thermodynamic control to the correct insulin fold. The selenoinsulin (Se-Ins) exhibited a bioactivity comparable to that of BPIns. Interestingly, degradation of Se-Ins with IDE was significantly decelerated (τ 1/2 ≈8 h vs. ≈1 h for BPIns). The lifetime enhancement could be due to both the intrinsic stability of the diselenide bond and local conformational changes induced by the substitution.
Figure 1
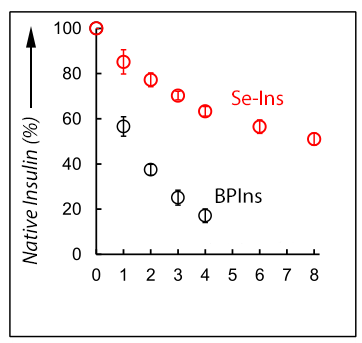
Figure 2
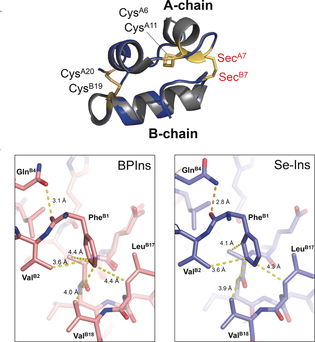
Figure 3
To learn more about this research, please view the full research report entitled “ Preparation of Selenoinsulin as a Long-Lasting Insulin Analogue ” at this page of the Angewandte Chemie website.
Related link
