Glycoproteins prepared through precision chemistry, elucidating erythropoietin sugar chains
Will lead to applications for the development of next generation biomedicine
Dr. Masumi Murakami, Mr. Tsuto Kiuchi, assistant professor Dr. Ryo Okamoto, associate professor Dr. Masayuki Izumi and Professor Dr. Yasuhiro Kajihara in Organic biochemistry laboratory, Department of Chemistry, Osaka University and Ms. Mika Nishihara and Dr. Katsunari Tezuka in Glytech Inc. successfully synthesized five kinds of erythropoietin 1 (EPO) derivatives by an organic synthetic approach and revealed the relationship between glycosylation 2 pattern and EPO bioactivity.
A protein having oligosaccharides is classified as a glycoprotein and this glycoprotein has been used for biological drugs. EPO is a typical glycoprotein drug and has been used for the treatment of chronic anemia. The addition of oligosaccharides to a bioactive protein is essential in order to enhance bioactivity, but the function of oligosaccharide is still unclear.
Cell expression method 3 , that is a biotechnology, can produces glycoproteins, but their oligosaccharides structures are always heterogeneous. Because of this heterogeneity, we cannot understand in details which oligosaccharide structure is essential for glycoprotein bioactivity.
The Murakami group demonstrated EPO having three homogeneous oligosaccharides at the native three glycosylation positions determined by genetic code during molecular evolution, which showed the most potent biological activity. The Murakami group also demonstrated that oligosaccharides play an important role in covering hydrophobic protein surfaces 4 in order to avoid hydrophobic protein-protein aggregation.
These research results will open a new research avenue not only to develop research on why glycosylation position was determined dependent on protein characteristic nature during the molecular evolution process but also to make potent as well as pure glycoprotein drugs in the future.
These research results have been published in Science Advances , 15 Jan 2016:
Vol. 2, no. 1, e1500678, DOI: 10.1126/sciadv.1500678
(http://advances.sciencemag.org), AAAS (American Association for the Advancement of Science).
Technical terms:
erythropoietin 1 : Erythropoietin (EPO) consists of 166 amino acids and has 3 asparagine linked oligosaccharides (N-glycan) and a serine linked oligosaccharide (O-glycan). N-Glycan concerns bioactivity of EPO. EPO has bioactivity to increase red blood cells.
glycosylation 2 : Incorporation of oligosaccharide with protein, lipid and some organic molecule is classified as glycosylation
Cell expression method 3 : mammalian cells, such as Chinese hamster ovary cells, have been used for production of glycoprotein drugs. We can prepare a desired glycoprotein though insertion of corresponding DNA into the cell. Glycosylation is automatically generated if DNA has the Asparagine-X-Serine/Threonine sequence (X: any amino acid except for proline). However, we cannot regulate oligosaccharide structures and this results in their heterogeneity.
Hydrophobic protein surfaces 4 : Some amino acids have aromatic rings and carbon chains which makes these amino acids difficult to dissolve into water. Hydrophobic protein surfaces are formed when these amino acids are located on the surface.
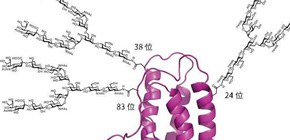
Figure A: Diverse oligosaccharide structure. In terms of 24 glycosylation position, one of these oligosaccharides may be incorporated. Biological synthesis can not regulate oligosaccharide structure.
Figure B: Structure of erythropoietin having three homogeneous oligosaccharides.
To learn more about this research, please view the full research report entitled “ Chemical synthesis of erythropoietin glycoforms for insights into the relationship between glycosylation pattern and bioactivity ” at this page of the Science Advances website.
Related Link
- Kajihara Laboratory , Graduate School of Science, Osaka University

