Encapsulated lithium ions increased reactivity of fullerenes 2400-fold
Successful clarification of intramolecular effects of lithium ion encapsulation
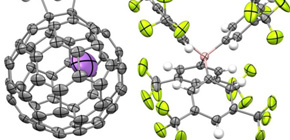
A group of researchers succeeded in measuring the reactivity of Li+-encapsulated [60]fullerene (Li+@C60), a world first.
• Graduate School of Science, The University of Tokyo -- MATSUO Yutaka , Professor
• Graduate School of Engineering, Osaka University -- KOKUBO Ken , Professor, IKUMA Naohiko , Professor, OSHIMA Takumi , Professor Emeritus
• Department of Information and Biological Sciences, Nagoya City University -- AOYAGI Shinobu , Professor
The results of their research on the Diels-Alder reaction revealed that, compared with empty C60, Li+@C60 reacted 2400-fold faster, a result which successfully clarified intramolecular effects of lithium ion encapsulation. The Diels-Alder reaction is used for chemical engineering and drug synthesis and is affected by steric effects and electronic effects. However, in this reaction, as the empty fullerene and the lithium ion encapsulated fullerene have the same configuration and volume, there were no differences in steric effects, thereby making possible to discuss only the electronic effects of encapsulated lithium ions.
This group's achievement will deepen understanding of this reaction and possibly become a guideline for designing new catalysts. The obtained product, lithium ion encapsulated fullerenes, will be used for organic photovoltaics, lithium cells, and capacitors as an organic functional material outperforming other fullerenes.
Abstract
We studied the kinetics of the Diels–Alder reaction of Li+-encapsulated [60]fullerene with 1,3-cyclohexadiene and characterized the obtained product, [Li+@C60(C6H8)](PF6–). Compared with empty C60, Li+@C60 reacted 2400-fold faster at 303 K, a rate enhancement that corresponds to lowering the activation energy by 24.2 kJ mol–1. The enhanced Diels–Alder reaction rate was well explained by DFT calculation at the M06-2X/6-31G(d) level of theory considering the reactant complex with dispersion corrections. The calculated activation energies for empty C60 and Li+@C60 (65.2 and 43.6 kJ mol–1, respectively) agreed fairly well with the experimentally obtained values (67.4 and 44.0 kJ mol–1, respectively). According to the calculation, the lowering of the transition state energy by Li+ encapsulation was associated with stabilization of the reactant complex (by 14.1 kJ mol–1) and the [4 + 2] product (by 5.9 kJ mol–1) through favorable frontier molecular orbital interactions. The encapsulated Li+ ion catalyzed the Diels–Alder reaction by lowering the LUMO of Li+@C60. This is the first detailed report on the kinetics of a Diels–Alder reaction catalyzed by an encapsulated Lewis acid catalyst rather than one coordinated to a heteroatom in the dienophile.

Figure 1
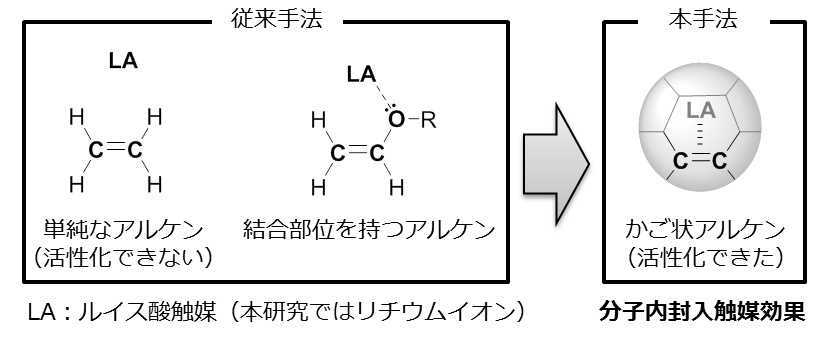
Figure 2
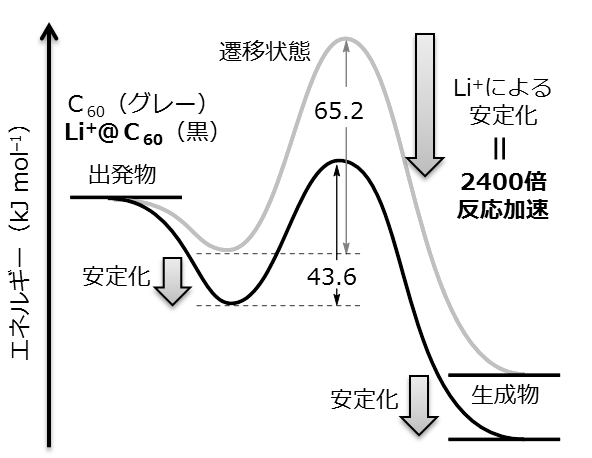
Figure 3
To learn more about this research, please view the full research report entitled " Kinetic Study of the Diels-Alder Reaction of Li+@C60 with Cyclohexadiene: Greatly Increased Reaction Rate by Encapsulated Li+ " at this page of the Journal of the American Chemical Society website.
Related links :
