It’s crystal-clear: Neutron beam uncovers reaction of the denitrification process in nature
Researchers use neutron structure analysis to understand an enzyme that may help equilibrate nitrogen cycling in nature
For over 100 years, chemists have applied amounts of nitrogen fertilizer to soils to help feed the world; however, this leads to eutrophication, an imbalance in the nitrogen cycle, one of the factors which causes destruction of the ozone layer. But now, researchers from Japan have experimentally verified the atomic-scale structure of an enzyme that may help to improve the environmental sustainability of this chemical reaction.
In a study published in Proceedings of the National Academy of Sciences , researchers from Osaka University used a technique called neutron diffraction crystallography to image the atom-by-atom architecture of an enzyme that belongs to a class known by the acronym CuNIR. If eutrophication progresses and excess nitrogen is present in the water and surrounding area, the large amount of plankton resulting from this excess will lead to water environmental problems, such as toxicity and odor, as well as various environmental problems, such as acid rain and ozone decomposition.
"Humanity needs to understand how nature performs this reaction so we can design an artificial reaction and perform it more efficiently," explains Tsuyoshi Inoue, a co-lead author of the study.
Previously, researchers haven't been able to see all of the atomic-scale detail of CuNIR enzymes, nor have they been able to avoid damaging these enzymes while imaging them. The Osaka University research team's imaging technique overcomes these limitations by using neutron diffraction. In doing so, the researchers experimentally verified theoretical calculations of the enzyme's structure.
These new experimental results clarified a long-standing, frustrating mismatch between lab work and calculations. "We confirmed details of copper binding and hydrogen bond dynamics that were predicted by theoretical calculations, but were obscured by experiments in the lab," explains Yohta Fukuda, senior author.
In addition to minimizing a global source of nitrogen pollution, the research team's findings may also be useful toward normalizing the nitrogen cycle. Inoue says: "Neutron diffraction crystallography may improve our understanding of enzymes that we can use to degrade nitrogen-containing pollutants throughout the environment."
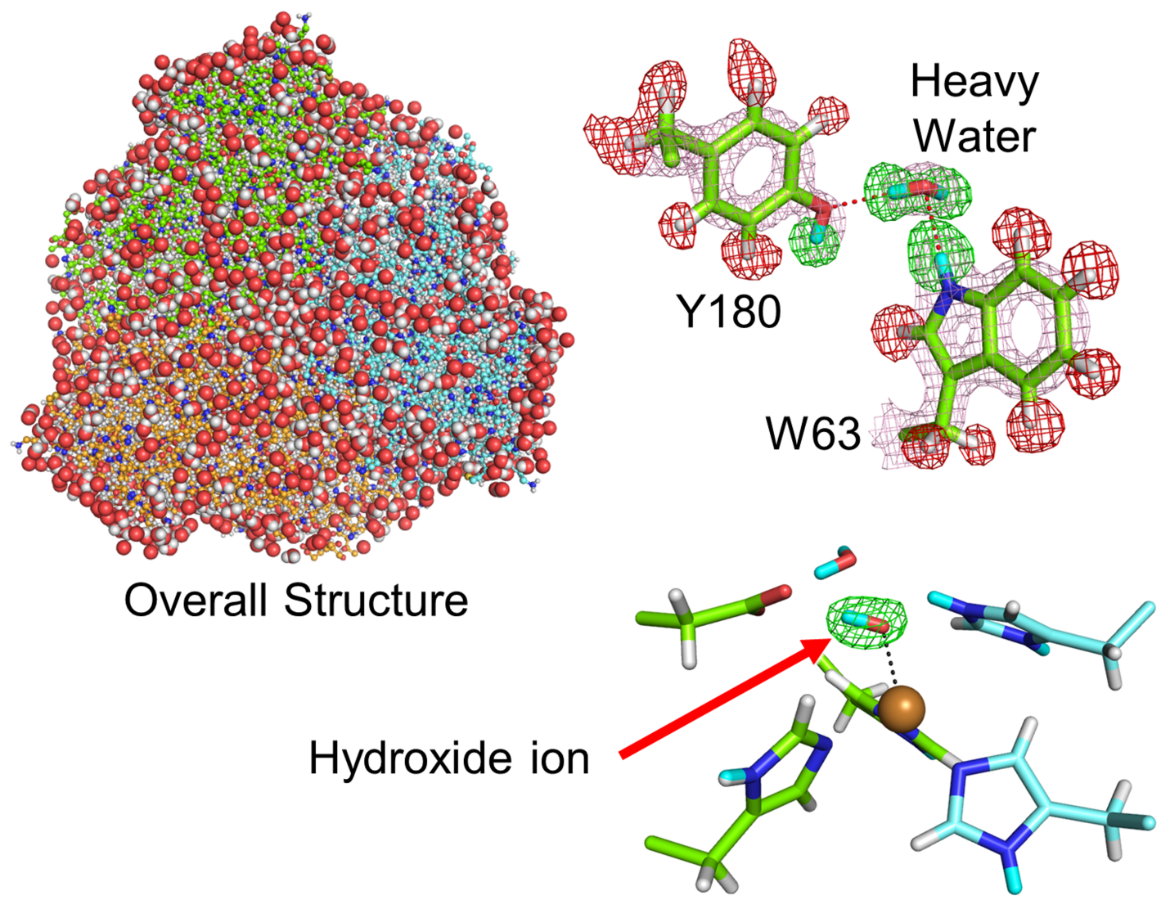
Fig. Neutron crystal structure of CuNIR : Because neutron crystallography can visualize detailed protein structures including hydrogen, the smallest atom in this world, one can distinguish a hydroxide ion, a key intermediate of the chemical reaction, from a water molecule.
The article, “High-resolution neutron crystallography visualizes an OH-bound resting state of a copper containing nitrite reductase” was published in Proceedings of the National Academy of Sciences at DOI: https://doi.org/10.1073/pnas.1918125117 .
Related links
