Researchers discover molecular light switch in photoreceptor cells
Researchers from Osaka University identify an enzyme that helps control vision in bright and dark spaces, with significant implications for the prevention of light-induced vision damage
How our eyes detect and respond to changes in light intensity is determined by specialized cells in the eye called photoreceptors. In addition to converting light into electrical signals, effectively allowing us to see, rod-shaped photoreceptors adapt to changes in light intensity to protect the eye from damage caused by excessive light exposure. However, in conditions such as retinitis pigmentosa, these cells do not work properly, leading to progressive vision loss. Now, researchers from Japan’s Osaka University have identified the protein responsible for the “switch” between light and dark adaptation.
In the study published this month in The EMBO Journal , the researchers describe how the enzymatic activity of the newly identified protein, Cul3-Klhl18 ubiquitin ligase, helps control the response of rod photoreceptors to changes in light intensity.
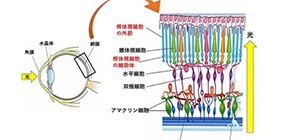
Rod photoreceptor cells contain a protein called transducin, which is part of the phototransduction cascade. “Under dark-adapted conditions, transducin is concentrated near the outer part of the cells,” explains lead author of the study Taro Chaya. “When light is detected, the transducin proteins move down into the inner section of the rod photoreceptor cells, modulating photosensitivity. However, previously no one has determined how this process is regulated.”
To identify genes involved in regulating photosensitivity, the researchers compared gene expression in healthy mice and those with defective photoreceptor cells. One gene, Klhl18 , stood out because it was predominantly expressed in the retina, specifically in the outer layer where photoreceptor cells are located. More importantly, the in vivo function of the gene product, Cul3-Klhl18 ubiquitin ligase, had been unclear.
To confirm the importance of Klhl18 , the researchers generated a Klhl18 -deficient mouse line and examined how the mice responded to changes in light intensity. Sure enough, the mice missing Cul3-Klhl18 ubiquitin ligase showed reduced responsiveness to light, and transducin remained in the inner section of rod photoreceptor cells. Importantly though, the Klhl18 -deficient mice were less susceptible to light-induced photoreceptor damage compared with their normal counterparts.
“We then wanted to know how Cul3-Klhl18 ubiquitin ligase interacts with transducin in the cells,” says senior author Takahisa Furukawa. “Transducin is moved around the cell by a protein called Unc119. When we studied the system more closely, we found that in the dark, Unc119 is tagged with something called ubiquitin and then degraded by the Cul3-Klhl18 ligase, allowing translocation of transducing to the outer part of photoreceptor cells. Upon light exposure, Unc119 is phosphorylated, preventing degradation and transducin migration.” These results suggest that therapies aimed at inhibiting Cul3-Klhl18 ubiquitin ligase may help treat degenerative retinal diseases such as age-related macular degeneration and retinitis pigmentosa.
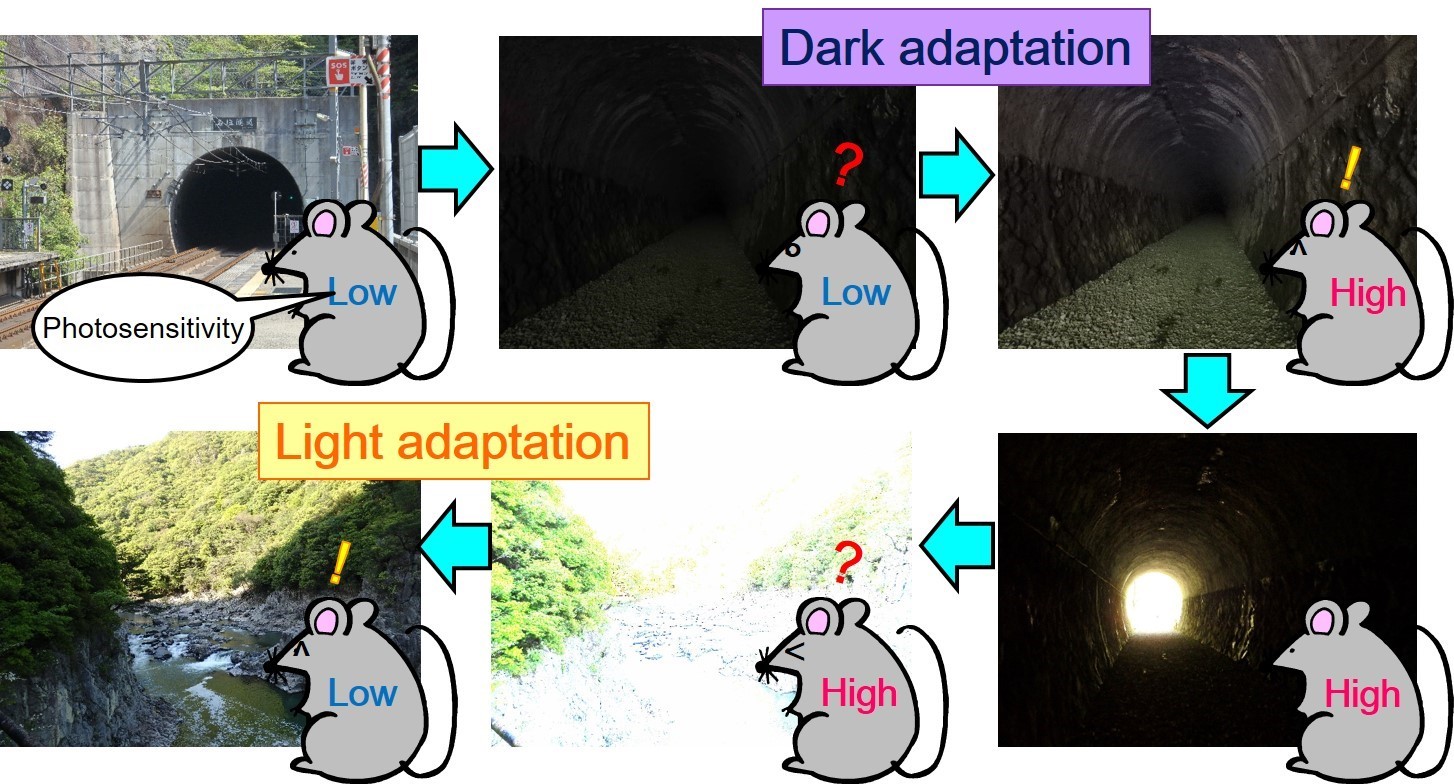
Fig.1 Light and dark adaptation
In low light, dark adaptation is achieved by increasing photosensitivity. Under bright conditions, light adaptation is achieved by decreasing photosensitivity.
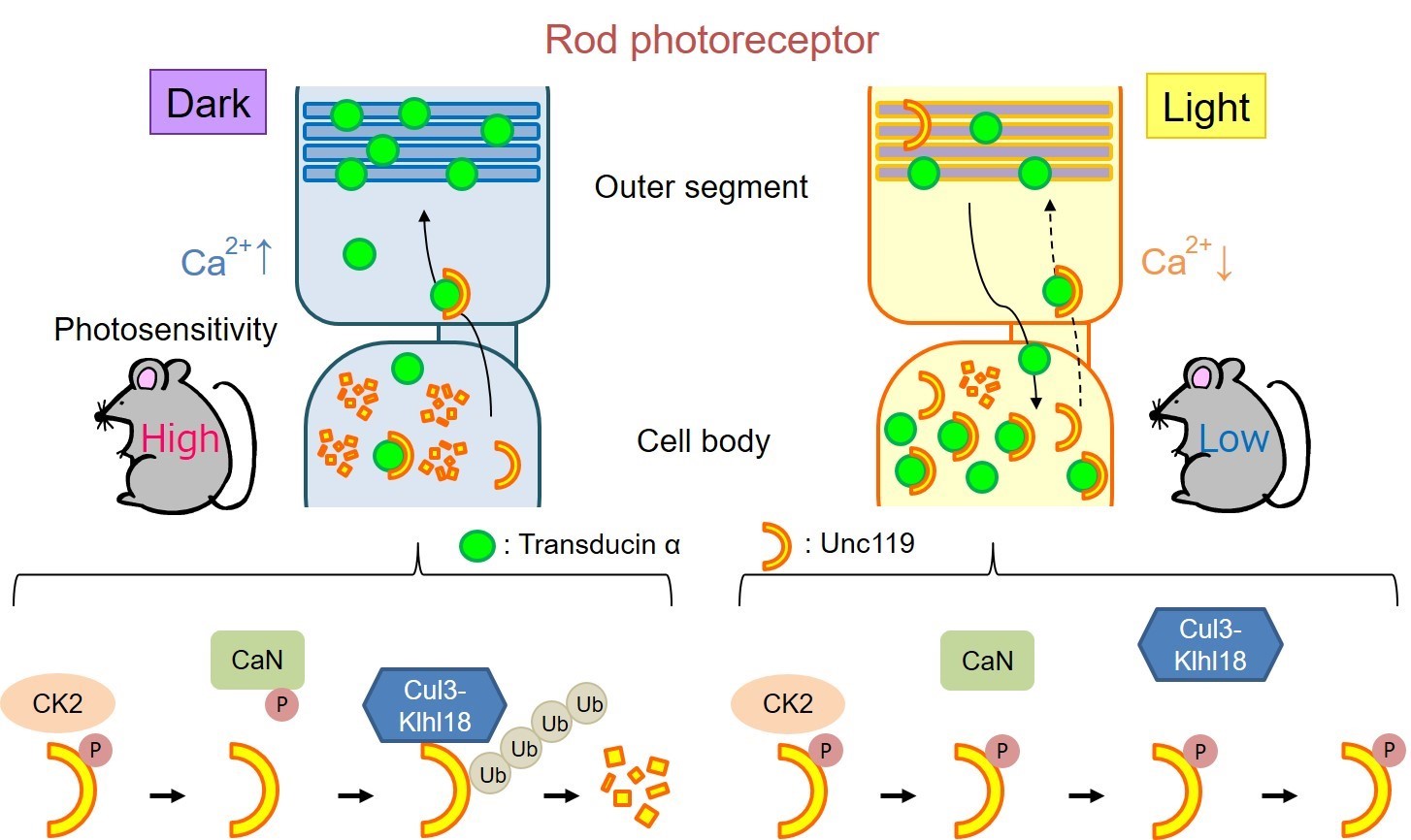
Fig.2 Summary of our results
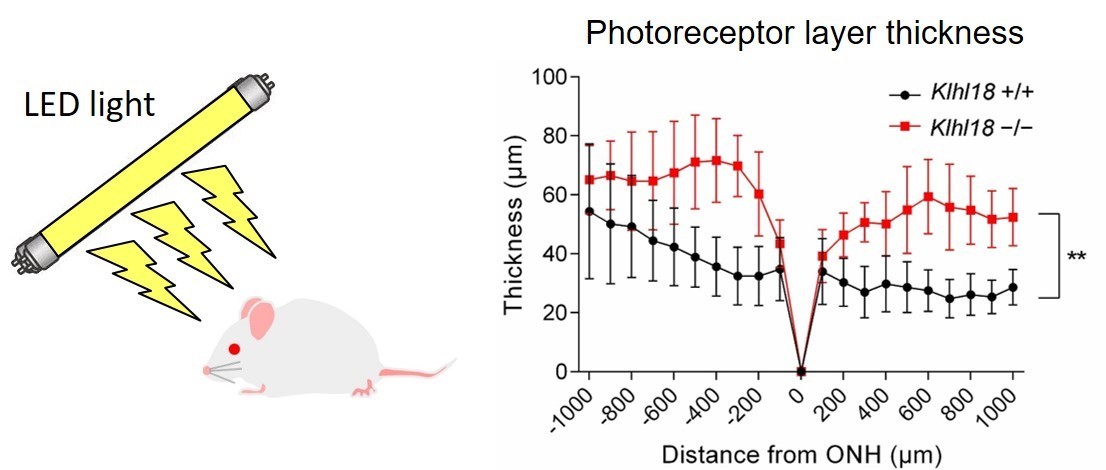
Fig.3 Klhl18 deficiency suppresses light-induced retinal damage
The article, “Cul3-Klhl18 ubiquitin ligase modulates rod transducin translocation during light-dark adaptation,” was published in The EMBO Journal at DOI: https://doi.org/10.15252/embj.2018101409 .
Related links
