Mechanism behind suppressing formation of aneuploid gametes clarified
A group of researchers led by Professor Akira SHINOHARA at the Institute for Protein Research discovered a cleavage-independent pathway to remove cohesin from chromosomes, clarifying the role of cohesin in the pathway and its mechanisms.
Cohesin is a protein complex that regulates the separation of sister chromatids during cell division in meiosis. By strictly controlling this pathway, gametes (the sperm and egg) with the correct number of chromosomes can be produced via meiosis while preventing aneuploidy.
It was thought that sister chromatids were held together by cohesion and were segregated during chromosomal division. In humans, eggs are arrested at an early stage of the first meiotic division in the ovary. The eggs resume meiosis by hormonal stimulus and most often only one egg will reach its mature state, capable of fertilization.
As women get older, their eggs age; that is, aneuploid eggs with an abnormal number of chromosomes become prevalent. Aneuploid eggs can cause trisomy, resulting in miscarriages and Down syndrome, which is a great risk associated with late birth. Chromosome cohesion decreases in human eggs with advanced maternal age, which was thought to be a cause of aneuploidy, but it was not well understood.
The group examined cohesin dynamics during meiosis by using budding yeast, whose chromosomes share a number of important features with human chromosomes and found:
(1) There is a cleavage-independent release of cohesin prior to the final cleavage-dependent removal of cohesin, and chromosomal division can be facilitated by strictly regulating this mechanism.
(2) Regulating the binding of cohesin to chromosomes is important for forming gametes with the correct number of chromosomes.
Thus, they clarified a mechanism for proteins involved in the regulation of cohesin dissociation from chromosomes at a molecular level.
The results of this study will lead to the clarification of causes of aneuploidy in eggs, causes of egg aging, and the development of drugs for diagnosis and prevention. It is thought that malfunction of cohesion, especially malfunction of the pathway for controlling cohesin, may cause abnormal chromosomal division by egg aging. In other words, regulation of this pathway through therapy or other means will enable treatment of aneuploidy.
Abstract
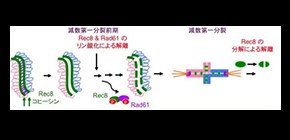
Sister chromatid cohesion on chromosome arms is essential for the segregation of homologous chromosomes during meiosis I while it is dispensable for sister chromatid separation during mitosis. It was assumed that, unlike the situation in mitosis, chromosome arms retain cohesion prior to onset of anaphase-I. Paradoxically, reduced immunostaining signals of meiosis-specific cohesin, including the kleisin Rec8, were observed on chromosomes during late prophase-I of budding yeast. This decrease is seen in the absence of Rec8 cleavage and depends on condensin-mediated recruitment of Polo-like kinase (PLK/Cdc5). In this study, we confirmed that this release indeed accompanies the dissociation of acetylated Smc3 as well as Rec8 from meiotic chromosomes during late prophase-I. This release requires, in addition to PLK, the cohesin regulator, Wapl (Rad61/Wpl1 in yeast), and Dbf4-dependent Cdc7 kinase (DDK). Meiosis-specific phosphorylation of Rad61/Wpl1 and Rec8 by PLK and DDK collaboratively promote this release. This process is similar to the vertebrate “prophase” pathway for cohesin release during G2 phase and pro-metaphase. In yeast, meiotic cohesin release coincides with PLK-dependent compaction of chromosomes in late meiotic prophase-I. We suggest that yeast uses this highly regulated cleavage-independent pathway to remove cohesin during late prophase-I to facilitate morphogenesis of condensed metaphase-I chromosomes.
Figure 1. Top, three step removal of cohesin during meiosis of the budding yeast. Bottom, possible model of cohesin release during late prophase-I by phosphorylation of Rec8 and Rad61, which may trigger the opening of the exit gate between Smc3 head and Rec8.
The article, “Meiosis-specific prophase-like pathway controls cleavage-independent release of cohesin by Wapl phosphorylation” was published in the PLOS Genetics at DOI: https://doi.org/ 10.1371/journal.pgen.1007851 .
Related links
- Laboratory of Genome-Chromosome Functions, Institute for Protein Research (link in Japanese)

