Study Identifies Molecule that Limits Excessive Expansion of Heart Muscle Cells
Identified protein that stops heart muscle cells from increasing in size when the heart is under stress
When the heart is subjected to stress, such as high blood pressure, it responds by expanding, both at the level of the whole organ or some of its chambers, and at the level of single cells. Although the swelling of heart muscle cells, called cardiomyocytes, has been investigated, understanding of the molecular mechanisms that promote and inhibit this has remained limited. Researchers centered at Osaka University have now identified a protein that restrains the speeding up of activity and expansion of cells that usually occur under stressful conditions. This novel finding explains how establishment of oversized cells can be prevented in heart muscle, which could lead to treatments to limit heart expansion that has been linked to heart failure.
Cardiac hypertrophy is when the heart muscle is placed under higher stress than normal and needs to develop greater bulk to deal with this. At the cellular level, cardiomyocytes are induced to increase size by speeding up production of proteins and other components. The production line from genes to proteins includes the intermediate of RNA, which forms an extra regulatory level determining the overall composition of proteins within a cell. Therefore, to understand the swelling of cardiomyocytes under stressful conditions due to increased production of proteins, it is necessary to clarify the overall regulation of RNA within these cells.
A new study by researchers centered at Osaka University has provided a major advance in this field by identifying the protein Btg2 as a global regulator of RNA within cardiomyocytes. By performing imaging of single cells, the induction of excessive expression of this protein within stressed cardiac tissue was found to reduce the size of cardiomyocytes, showing that Btg2 is a factor that limits cardiac hypertrophy.
“We first focused on the targets of the protein Myc, which is known to increase the size and anabolic activity of cardiomyocytes,” corresponding author Shuichiro Higo says. “We found that Btg2 was especially strongly induced by Myc, but that these two proteins had opposite effects on the level of RNA in these cells. This suggests they are on opposing sides of a system regulating protein production and cell size.”
Application of single-cell imaging in this study enabled the team to identify where Btg2 was active within the cell, which was combined with functional analysis to determine the mechanisms by which it induced a reduction in global RNA level. The findings showed that Btg2 interacts with cellular machinery that breaks down RNA, which explains its association with reduced protein production and, thus, smaller cells.
“We now have a much better understanding of the mechanisms by which heart cells can not only expand in response to stress, but also limit this expansion,” Higo says. “We may be able to harness these mechanisms to reduce some of the problems associated with long-term cardiac hypertrophy and heart disease.”
Abstract
Under hypertrophic stimulation, cardiomyocytes enter a hypermetabolic state and accelerate biomass accumulation. Although the molecular pathways that regulate protein levels are well-studied, the functional implications of RNA accumulation and its regulatory mechanisms in cardiomyocytes remain elusive. Here, we have elucidated the quantitative kinetics of RNA in cardiomyocytes through single cell imaging and c-Myc (Myc)-mediated hypermetabolic analytical model using cultured cardiomyocytes. Nascent RNA labeling combined with single cell imaging demonstrated that Myc protein significantly increased the amount of global RNA production per cardiomyocyte. Chromatin immunoprecipitation with high-throughput sequencing clarified that overexpressed Myc bound to a specific set of genes and recruits RNA polymerase II. Among these genes, we identified Btg2 as a novel target of Myc. Btg2 overexpression significantly reduced cardiomyocyte surface area. Conversely, shRNA-mediated knockdown of Btg2 accelerated adrenergic stimulus-induced hypertrophy. Using mass spectrometry analysis, we determined that Btg2 binds a series of proteins that comprise mRNA deadenylation complexes. Intriguingly, Btg2 specifically suppresses cytosolic, but not nuclear, RNA levels. Btg2 knockdown further enhances cytosolic RNA accumulation in cardiomyocytes under adrenergic stimulation, suggesting that Btg2 negatively regulates reactive hypertrophy by negatively regulating RNA accumulation. Our findings provide insight into the functional significance of the mechanisms regulating RNA levels in cardiomyocytes.
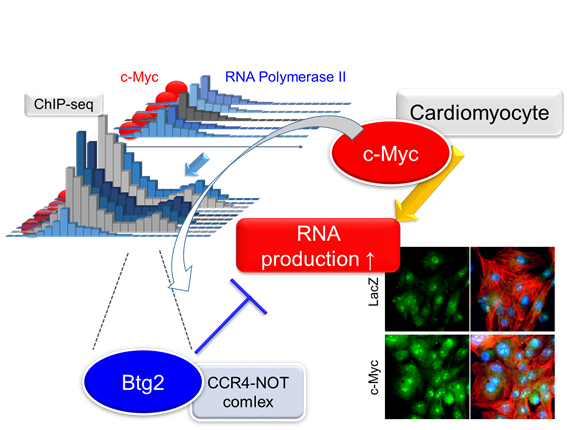
Identification of the regulating factor of RNA amount in cardiomyocytes using whole genome binding profiling
To learn more about this research, please view the full research report entitled “ Btg2 is a Negative Regulator of Cardiomyocyte Hypertrophy through a Decrease in Cytosolic RNA ” at this page of the Scientific Reports website.
Related link
