Synthesis of Double Helicenes Achieved in Just Two Steps
Just two steps!?
A group of researchers developed a method for easily synthesizing double helicenes by using N-heteropentacene, an organic semiconductor material.
- Applied Chemistry, The Graduate School of Engineering, Osaka University -- SAKAMAKI Daisuke (Researcher), SEKI Shuhei (Professor)
- Molecular Design & Engineering, Graduate School of Engineering, Nagoya University -- KUMANO Daisuke ( 2nd year student of master's program ) YASHIMA Eiji ( Professor)
Helicene shows different optical features depending on its helical orientation. As a candidate for new optical material, research regarding its application has been conducted. Helecene shows promise for use as a circularly-polarized emission material as well as a semiconductor material in which electron mobility can be changed by applying pressure. This group's achievements will possibly lead to application to organic light-emitting (OLE) and organic field-effect transistor (OFET) used for illumination, TV, and displays.
Abstract
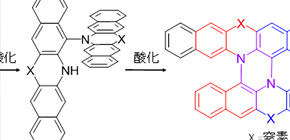
In this work, we report the synthesis of novel double N-hetero[5]helicenes composed of two nitrogen-substituted heteropentacenes by using tandem oxidative C-N coupling via cruciform heteropentacene dimers. The synthetic method is very facile enough to make a double helicene taking only two steps from commercially available naphthalene derivatives. These double N-hetero[5]helicenes have larger torsion angles in the fjord regions compared to usual [5]helicene, and the optical/electrochemical measurement revealed the significant increase of the electronic communication between two heteropentacene moieties of the double helicenes than their crucifor dimers. We succeeded in the optical resolution for one of the double helicene, and the stability toward racemization was remarkably higher than usual [5]helicene. The synthetic strategy proposed in this paper could be versatile and widely applicable to the preparation of double helicenes from other N-containing π-conjugated planar molecules.
For reference
In this article, 3D pi-conjugated molecular bricolages: double [5]helicenes were designed and synthesized by using tandem oxidative C-N coupling via cruciform heteropentacene dimers. The synthetic method employed in the present study is very facile to give a double helicene, required only two steps. Electronic structures of the double [5]helicenes are unique, suggesting considerable electronic communication between two azaacene units, which have been well demonstrated not only by a variety of spectroscopic and electrochemical measurements but also by the DFT and TD–DFT calculations.
Especially, the oxidation of the double helicenes showed clear spectroscopic signature of charge resonance interaction among azaacene moieties, and hence delocalization of positive charges and spins over the moieties is suggestive as the electronic conductive nature of the present molecular systems. It should also be noted that the racemates of double [5]helicenes were successfully resolved optically, leading to the respective enantiomers. Surprisingly, the enantiomers exhibited high enough stability, and the racemization of one of the helicenes were well suppressed without changes in the enantiopurity under 100 °C over 10 h in the solutions phases.The synthetic strategy of the double helicenes proposed in this paper is thought to have high generality and be applicable to the various heteroacenes. So far, this is one of the simplest and the most versatile approach to give the Double Helicenes.
Fig. 1. Synthesis of double heterohelicenes via cruciform heteroacene dimers by tandem oxidative coupling.
Fig. 2. Crystal structures of double helicenes with opposite helical directions.
To learn more about this research, please view the full research report entitled " A Facile and Versatile Approach to Double N-Heterohelicenes: Tandem Oxidative C-N Coupling Method of N-Heteroacenes via Cruciform Dimers " at this page of the Angewandte Chemie International Edition website.
Related Link
