Clarification of how inflammation causes nerve damage in multiple sclerosis (MS)
The Repulsive Guidance Molecule-a (RGMa) plays a role in neurodegeneration
A group of researchers led by YAMASHITA Toshihide , Professor, Department of Molecular Neuroscience, Department of Anatomy, Graduate School of Medicine, Osaka University, have discovered a way in which multiple sclerosis (MS) damages the central nerve system (CNS).
MS is an intractable disease that causes inflammation in the CNS through immune response abnormalities, damaging nerves, and producing severe symptoms such as limb paralysis, abnormalities in sensation, and visual impairment. It has been known that in MS immune cells invade the CNS, causing inflammation, and that that causes neurodegeneration, but how inflammation causes nerve damage has been unknown.
Using mice models of encephalomyelitis similar to MS, this group of researchers found that the Repulsive Guidance Molecule-a (RGMa) plays a role in neurodegeneration from inflammation and that certain immune cells caused neurodegeneration through expression of RGMa.
In mice experiments, this group succeeded in reducing neurodegeneration in encephalomyelitis by administrating a neutralizing antibody that reduced the activity of RGMa. This group differentiated helper T cells from the spleen of mice and checked the expression level of RGMa and found that one type of T cells, interleukin-17-producing CD4 + T cells (TH17), strongly expressed RGMa.
Administration of antibodies capable of preventing RGMa improved the situation of limb paralysis in mice with encephalomyelitis caused by transplanted TH17 cells. The administration of antibodies also reduced neurodegeneration in inflamed areas in the spinal marrow of these mice. These results showed that RGMa expressed in TH17 cells played a role in neurodegeneration. Although nerve cells cultivated with TH17 cells died, the death of nerve cells cultivated with RGMa-neutralizing antibodies was not observed in a culture solution with TH17 alone.
These results demonstrate that TH17 damages nerve cells through the RGMa-mediated direct contact. This group has clarified how TH17 damaged nerves and that preventing RGMa can be an effective method of treatment for neurodegeneration in MS. In recent years, TH17 has been found to be involved not only in MS, but also in cranial nerve diseases such as optic neuromyelitis and Alzheimer's Disease. The clarification of the role of TH17 in cranial nerve diseases will possibly lead to the development of effective treatment methods in the future.
Abstract
Multiple sclerosis (MS) is a chronic autoimmune disease characterized by inflammation, demyelination, and neurodegeneration in the CNS. Although it is important to prevent neurodegeneration for alleviating neurological disability, the molecular mechanism of neurodegeneration remains largely unknown. Here, we report that repulsive guidance molecule-a (RGMa), known to regulate axonal growth, is associated with neurodegeneration in experimental autoimmune encephalomyelitis (EAE), a mouse model of MS. RGMa is highly expressed in interleukin-17-producing CD4 + T cells (Th17 cells). We induced EAE by adoptive transfer of myelin oligodendrocyte glycoprotein (MOG)-specific Th17 cells and then inhibited RGMa with a neutralizing antibody. Inhibition of RGMa improves EAE scores and reduces neuronal degeneration without altering immune or glial responses. Th17 cells induce cultured cortical neuron death through RGMa-neogenin and Akt dephosphorylation. Our results demonstrate that RGMa is involved in Th17-cell-mediated neurodegeneration and that RGMa-specific antibody may have a therapeutic effect in MS.
Figure 1. Th17 cells highly express RGMa.
Helper T cells are classified into Th0, Th1, Th17 and regulatory T cells by their functions. Right graph indicates the expression levels of RGMa in the corresponding helper T cell. *** means significant differences.
Figure 2. The severity of encephalomyelitis is ameliorated by RGMa antibody injection
Encephalomyelitis was induced by adoptive transfer of Th17 cells which are activated in the CNS. Vertical axis indicates the level of encephalomyelitis severity assessed by behavior test, and horizontal axis means the days after transfer of Th17 cells. ** means significant differences.
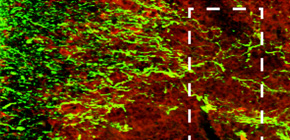
Figure 3. Neurons in the inflammatory site of encephalomyelitis mouse
Above image represents the immunohistochemical staining for axonal neurofilament (green) and inflammatory site (red) in spinal cord of encephalomyelitis mouse. Axons in inflammatory site are severely injured. Below images represent the immunohistochemical staining for axonal neurofilament (red) in spinal cord of healthy mouse and encephalomyelitis mice treated with control IgG or RGMa antibody. Axonal degeneration was suppressed by treatment with RGMa antibody.
Figure 4. Th17 cells directly damage neurons through RGMa.
Neurons and T cells were co-cultured for 24 hours and dead cells were stained (red). Although neurons died when co-cultured with Th17 cells, the addition of RGMa antibody suppressed the neuronal cell death. The conditioned medium from Th17 cells did not induce neuronal cell death. These results suggest that the direct interaction is required to induce neuronal cell death by Th17 cells. *, *** mean significant differences.
Figure 5. Th17 cells contact with neurons in inflammatory site and induce neurodegeneration through RGMa.
The pathogenic Th17 cells infiltrate into the CNS and exert neurotoxic effects through high expression of RGMa. To suppress neurodegenerative signals such as RGMa is an important therapeutic strategy to treat neurodegeneration in MS.
To learn more about this research, please view the full research report entitled " Repulsive guidance molecule-a is involved in Th17 cell-induced neurodegeneration in autoimmune encephalomyelitis " at this page of the Cell Reports website.
Related Link
